Zeliha Alboru, a third-year Biomedical Engineering student at Istinye University, was inspired to seek a solution for vitiligo after witnessing her father’s long-standing battle with the disease. Together with fellow university students İlayda Erdoğan and Aleyna Erdoğan, Alboru developed a Secure and Efficient IoT-Integrated UVB LED Phototherapy Device designed to treat vitiligo. The device, developed under the academic supervision of Istinye University Biomedical Engineering faculty member Dr. Aytaç Durmaz, introduces a new, more accessible approach to the treatment of the condition.
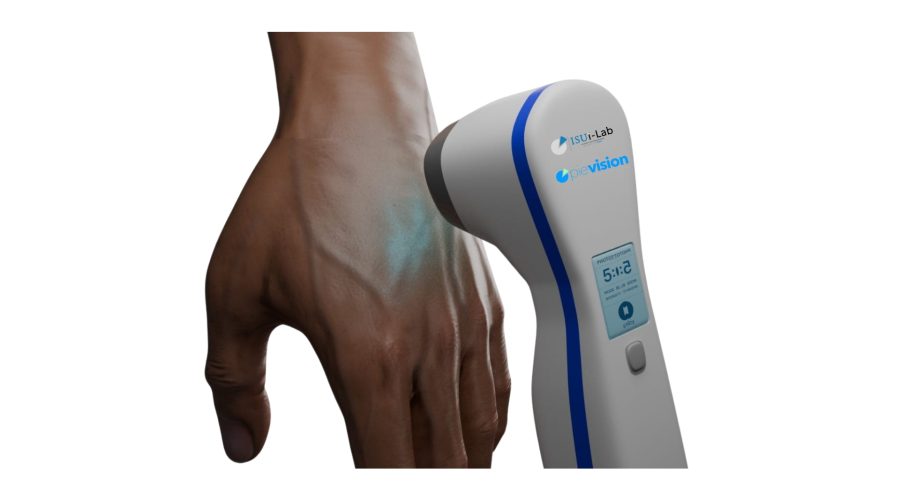
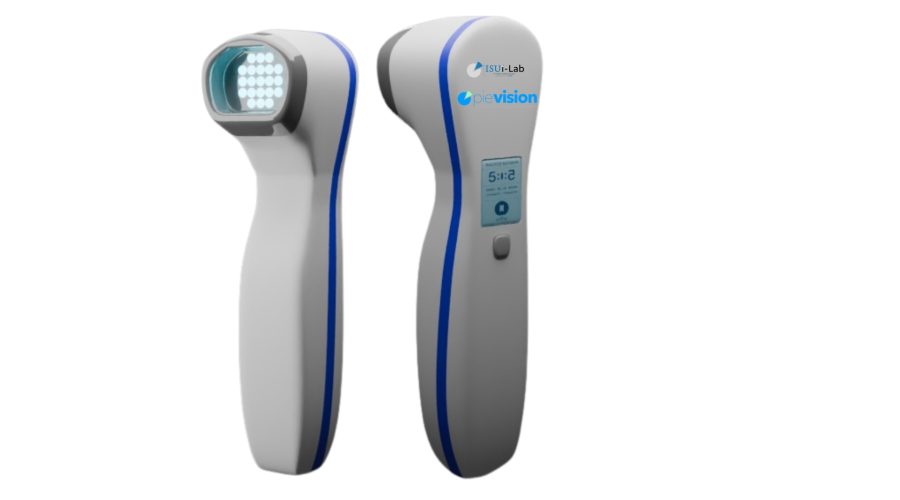
Traditionally, vitiligo treatments have relied on large-scale devices. However, the newly designed device offers a more portable and user-friendly alternative. It emits controlled UVB light in specific modes and durations, enabling targeted treatment. Unlike traditional phototherapy units, which expose the entire body to radiation, this device allows for localized application, minimizing exposure and maximizing comfort.
What Is Vitiligo?
Vitiligo is a condition caused by the death or damage of melanocytes—the cells responsible for skin pigmentation—leading to the appearance of white patches on the skin. Commonly referred to as the “white spot disease” or “ala disease” in Türkiye, vitiligo remains without a definitive cure. Zeliha Alboru, whose father also suffers from the condition, set out to create a more comfortable and practical solution for patients. The goal of the project is to improve treatment accessibility and effectiveness for those affected by vitiligo.
The project is being conducted under the academic guidance of Dr. Aytaç Durmaz and aims to ensure that the device is developed in compliance with clinical safety standards and medical device regulations, particularly MDR (Medical Device Regulation). The device’s compact design allows for convenient personal use and improved accessibility compared to traditional equipment.
“We Aim for Widespread Use in Dermatology”
Speaking about the project’s significance and its potential medical applications, Dr. Aytaç Durmaz stated:
“Supported by TÜBİTAK’s 2209-B University–Industry Cooperation Program, the project is receiving technical mentorship and infrastructure support from Pievision Technology in the development of the device’s software and electronic hardware. This collaboration is a successful example of university–industry integration and contributes significantly to the development of user-centered, innovative medical devices. Both Istinye University’s i-Lab and Pievision have expressed their commitment to supporting high-tech innovation and ongoing collaboration. Once advanced clinical trials are completed, we aim to make the device widely available for clinical use in dermatology.”
“73% of Patients Are Female”
Alboru cited data from a 2012 study led by Karadeniz Technical University, noting that vitiligo affects approximately 1–4% of the global population, and around 0.15% of the population in Türkiye who visit dermatology clinics. She explained:
“Although the exact cause of vitiligo is still unknown, the condition is observed more frequently in individuals with darker skin tones, and 73% of reported cases are women. The earliest onset has been recorded at age 3 and the latest at 54, though there have even been reports of the condition appearing at age 81. While the disease itself is not hereditary, genetic predisposition plays a role, with 20% of patients having a family history.”
“The Cause of the Disease Is Still Unclear”
Alboru further elaborated on the causes and treatment approaches:
“Vitiligo is fundamentally the result of melanin pigment in skin cells not functioning properly or dying. Though the exact reason is unknown, phototherapy has emerged as a prominent treatment method. Controlled application of UV light to depigmented areas has been shown in clinical studies to activate melanin production and improve pigmentation. Literature indicates treatment effectiveness rates ranging from 40% to 100%.”
“We Aim to Make the Device Safe and User-Friendly”
Alboru emphasized the importance of safety and education in the use of the device:
“Portable NB-UVB has been found to be safe and well-tolerated, but it requires guidance and support from medical professionals. Overexposure to UVB light can cause adverse effects such as sunburn, photoaging (loss of skin elasticity), eye damage, and even DNA damage. Therefore, it is crucial that treatment is administered under controlled parameters—ideally with medical supervision.”
“At Least One Year of Use Is Needed for Results”
On the expected frequency and duration of use, Alboru explained:
“The final response to treatment depends not on session frequency, but on the total number of sessions. Evaluating the effectiveness of NB-UVB phototherapy typically requires at least six months (48 sessions), and optimal results may take up to a year. Future cellular and clinical studies will help define the ideal treatment duration. This forms a key part of the next phase of our project.”
“We Are Working to Complete Clinical Trials”
Describing the current stage of development, Alboru shared:
“We are currently in the software development phase. Once the software is finalized, hardware components will be assembled to create the first prototype. Developing a medical device is a time-intensive, cost-heavy process that demands high precision and attention.”